
-
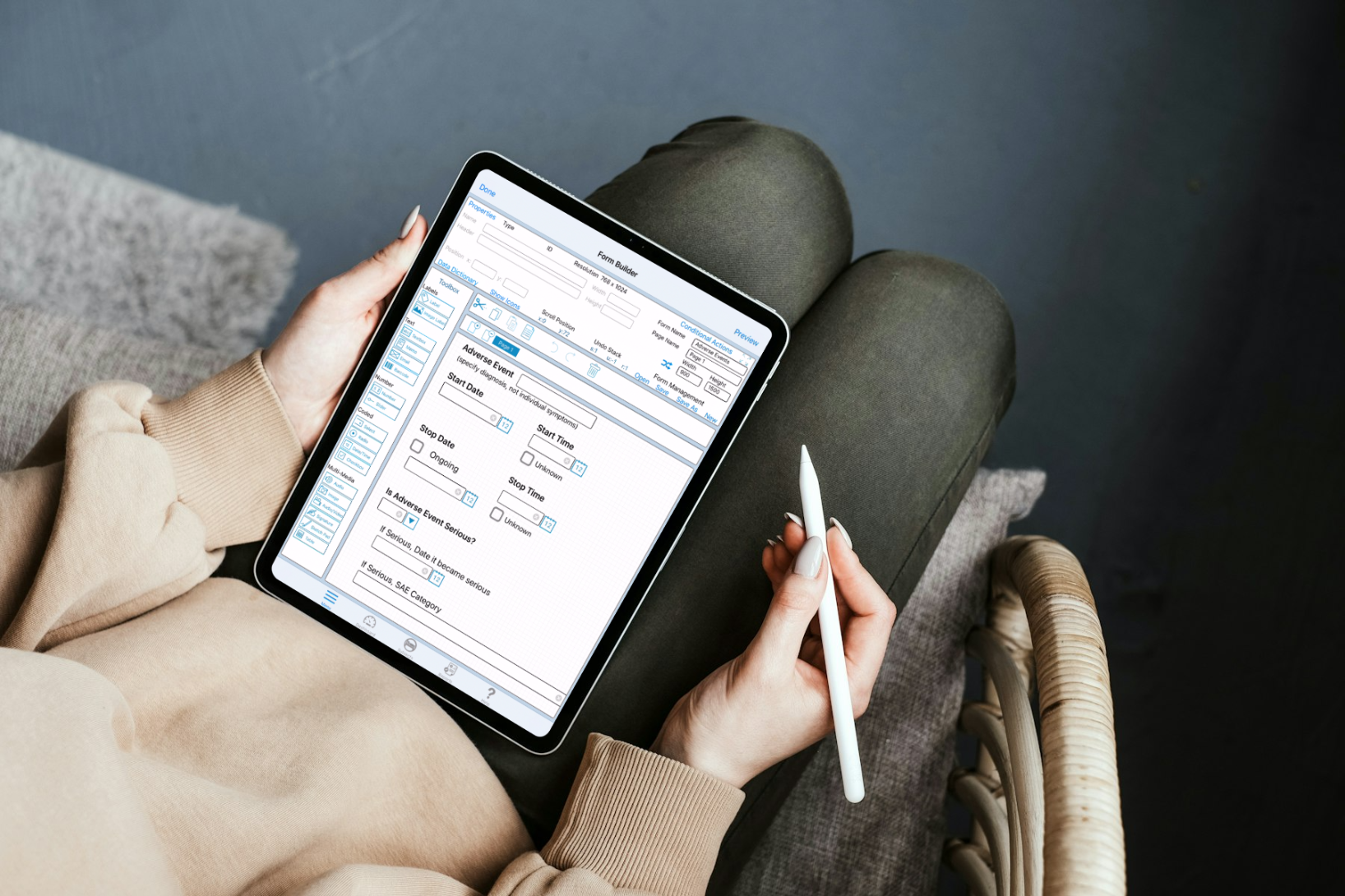
Build Better Studies Faster: 3 Advantages of Mobile eClinical Platforms
The landscape of clinical research is undergoing a rapid digital transformation. Data can be collected in ways not imagined just…
-

It Might Be Time to Move on from Your Legacy EDC
The tools and technologies we rely on to capture and manage clinical trial data are evolving at a rapid pace….
-

Finding Efficiency – Top Three Benefits of Electronic Data Capture (EDC)
Whether you’re a first-time user of electronic data capture software or you’re an experienced user looking to improve your processes, this post provides some helpful insights on the benefits of EDC systems.
-

Electronic Data Capture – The Basics
In a time with lots of hype surrounding decentralized clinical trial (DCT) solutions, let’s not lose sight of the importance of a dependable and secure electronic data capture (EDC) system. This blog post walks you through the basics to keep in mind when selecting an EDC platform for your studies.
-

Making Data Exchange Feel Easy – Simplifying the Data Journey from EHR to EDC
Demystifying and providing a solution for EHR to EDC interoperability has been a mission for CDS for the past four years. In this blog post, we break down various interoperability challenges and how the architecture of your eClinical software can be a game-changer.
-

It’s Your Data: Open API Gives You Better Access and the Freedom to Build and Manage Your Studies, Your Way
Unlike some providers in the eClinical space, at CDS we do not hold your data hostage when you conduct your studies in TrialKit. In fact, TrialKit is driven by an open API that gives users true freedom and access to their data – whenever they need it. Read on to learn how we do this…
-

A Glimpse into 2021: What to Expect from Crucial Data Solutions and TrialKit
CDS has many innovative updates in store for TrialKit in 2021. Check out this blog post for a quick glance at what’s to come to the platform in the upcoming year.
-
TrialKit, Native App for EDC and ePRO, to be Available for Android Devices in September
Fully-featured eClinical app, TrialKit, will soon be released on Google Play by Crucial Data Solutions, making clinical data collection and management possible on the majority of smartphones available today.
-
Rethinking the Need for “Signatures” in EDC Systems
Is there a more secure way to review and approve the data that’s collected on case reports forms? In this post, we examine the value and purpose of the traditional signature used in an EDC system.
-
All Your Research in One Place, Accessible Everywhere
The traditional methods of clinical data management tend to be disjointed, filled with tedious manual work and prone to human error. Our VP Product Development, Cody Wilke, explains how with the right EDC system, you can collect and access data from any device, any time.