The landscape of clinical research is undergoing a rapid digital transformation. Data can be collected in ways not imagined just ten years ago, and technology is now expanding patient access to research, breaking down geographic barriers, moving beyond wholly site-based studies to decentralized clinical trials (DCTs), and reshaping what modern trials look like.
Clinical trial professionals are evolving beyond the old model of working in office cubes tied to their computers. Modern studies can’t be managed effectively solely during “office hours.” And today’s professionals want to be able to live their lives – in and out of work – without sacrificing access to study data, because they know the importance of acting quickly when issues pop up.
Flexible eClinical platforms enable researchers to create and customize how they gather, manage, and monitor trial data on their preferred devices, ranging from smartphones to tablets to desktop computers. This approach not only aligns with the digital preferences of younger generations of research professionals but also promises to enhance the efficiency, accuracy, and scalability of modern and future clinical trials.
The Rise of Device-Agnostic Platforms in Clinical Research
The shift toward device-agnostic platforms in clinical studies reflects broader technological trends and a generational shift in workplace culture and technology preferences. Young professionals, often referred to as digital natives, are accustomed to seamless technology experiences that offer flexibility and instant connectivity. Additionally, they want the freedom to work remotely when needed. By incorporating these expectations into clinical research, the industry can better utilize the innovative potential of its workforce.
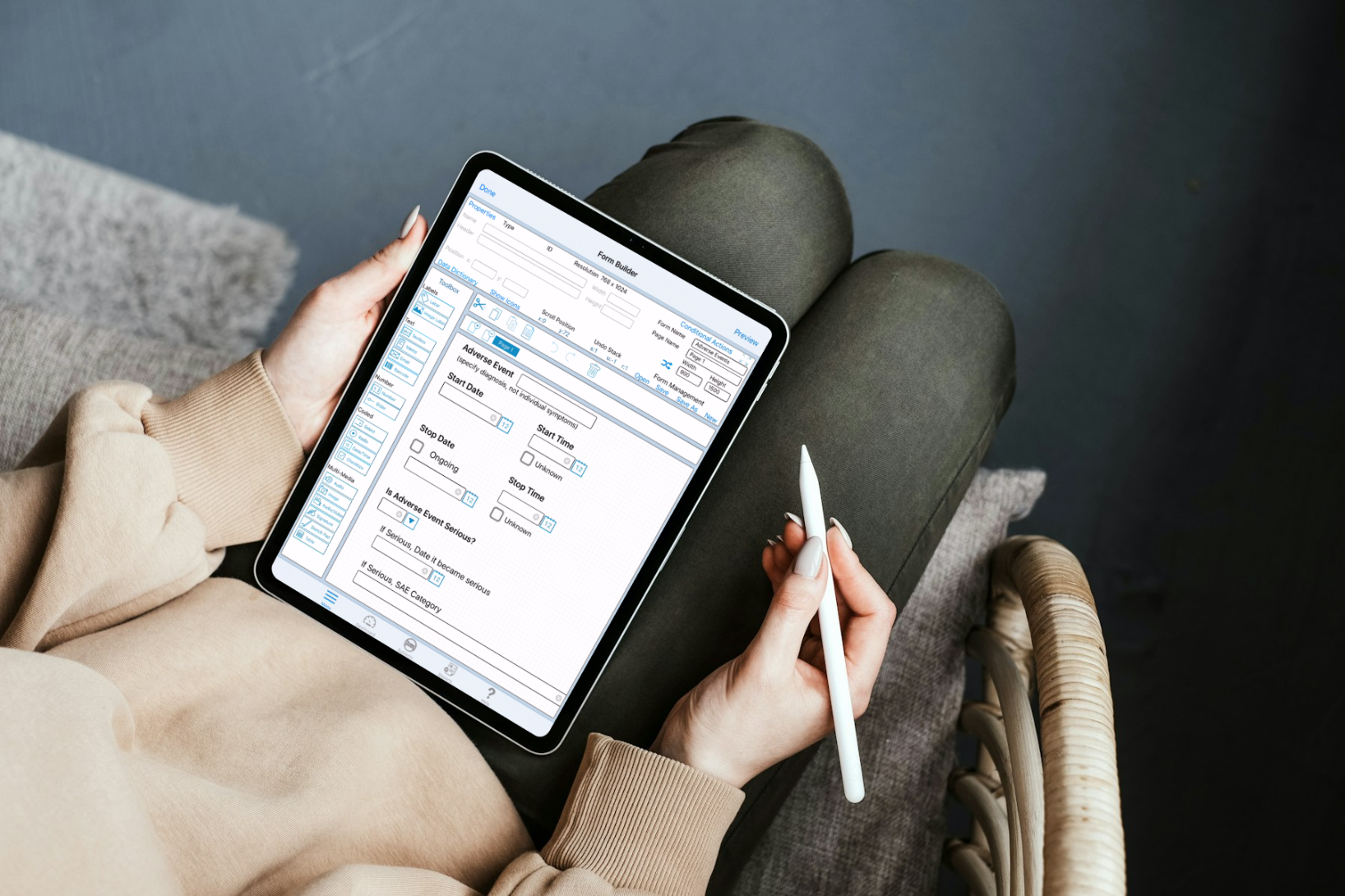
Advantage 1: Build Out Study Forms However You Want
Smart devices like iPads and phones are necessary parts of our lives now. They have become invaluable tools for managing our day-to-day activities. You can also easily implement them for many clinical trial study build functions. For example, using a tablet or similar device to build or edit study forms has several advantages:
- Accessibility: Researchers can access and modify study data anytime and anywhere, which is crucial for studies that involve multiple locations or remote monitoring.
- Real-time Updates: Make changes to study forms on-the-fly, allowing for quick adaptations to new regulations, scientific insights, or operational requirements.
- User Engagement: Smart devices are intuitive for younger generations, which can lead to higher engagement rates among researchers. It also has the potential to improve the accuracy and completeness of data capture.
Advantage 2: Easier Study Tool Configuration
Mobile platforms like TrialKit that support a wide array of configurable tools for managing different aspects of clinical studies further enhance the flexibility and capability of research platforms. Researchers can:
- Customize Data Collection Methods: Whether it’s quantitative data from electronic case report forms (eCRFs) and eDiaries or qualitative data from interviews, researchers can configure tools to best suit the data type.
- Integrate External Data Sources: Easy integration with electronic health records (EHRs), wearable technology, and other IoT devices ensures comprehensive data collection.
- Adapt to Study Needs: Tools can be scaled or adjusted depending on the phase of the trial or specific requirements. This ensures the application grows with the study.
Advantage 3: Drag and Drop to Simplify Study Design
The ability to drag and drop study components is more than a convenience—it’s a transformative feature for clinical trial design that enables:
- Ease of Use: Simplifies the process of study design, especially for those without extensive technical expertise.
- Flexibility in Study Design: Quickly rearrange components, test different study setups, and refine workflows without the need for complex programming.
- Rapid Prototyping: Studies can be prototyped and tested in a virtual environment, allowing for faster iteration and innovation.
Setting the Stage for Efficient Direct Data Capture
The ability to build and interact with study data from a centralized cloud-based platform available on any device may be key to solving one of the industry’s looming challenges: large-scale direct data capture. eClinical platforms that provide this kind of flexibility, like TrialKit, will play a role in direct data capture that is efficient and less prone to errors.
Applications that allow for direct entry of data into the system from the point of first interaction (e.g., patient data from surveys entered directly into a tablet, or data from wearable sensors) reduce the risk of transcription errors and data loss. Moreover, this directness accelerates the pace at which data can be analyzed and reviewed.
Nimble platforms are necessary as researchers hope to maintain the ability to perform real-time data analysis amid the growing number of data points and sources. These real-time analytics can provide immediate insights into patient outcomes or trial efficacy, enabling quicker decision-making and adaptive trial designs. Platforms like TrialKit make it simple to add new tools, including those powered by artificial intelligence and machine learning, to help get from data entry to actionable insights fast.
Better Study Build Tools = Better-Built Studies = Better Data
By enabling researchers to easily build and modify study parameters from any device—be it a tablet, phone, or computer—flexible eClinical platforms democratize and streamline the process of clinical trial management.
The flexibility to work from any device is not just a matter of convenience; it changes how quickly a study can pivot in response to new data, regulatory changes, or logistical challenges. Moreover, such platforms, supported by secure cloud accessibility, ensure that every team member, regardless of their location or preferred device, has the same tools and information, enhancing collaboration and consistency across the study.
For stakeholders at all levels—sponsors, CROs, and site teams—platforms like TrialKit simplify the task of accessing and sharing crucial study information. By centralizing data and workflows in one intuitive platform, these tools ensure that every stakeholder has what they need to do their jobs well, including making informed decisions, monitoring progress, and identifying potential risks and issues before they can derail a trial.
The flexibility provided by device-agnostic applications and data platforms in clinical research is not a trend nor a gimmick. Rather, it is a significant shift towards more dynamic and responsive study designs – the precise kinds of designs which are becoming more common as studies increase in complexity. Younger generations of researchers are already seeking out solutions that match the ways they want to work.
TrialKit is a solution that bridges the gap between users who want an entirely mobile experience and those who are most comfortable at a traditional work station. As the tools and strategies used to collect, manage, and monitor clinical trial data become more sophisticated, we must be able to react quickly to changing research needs. Flexible and accessible platforms can help meet the needs of both current and future studies, regardless of what new innovations, data sources, and strategies emerge.
Want to learn more about how to use TrialKit’s mobile study build features? Contact us today.