Electronic Trial Master File (eTMF) Software for Streamlined Document Management
Manage inspection-ready clinical trial documents with confidence for faster study execution with TrialKit’s electronic trial master file system.

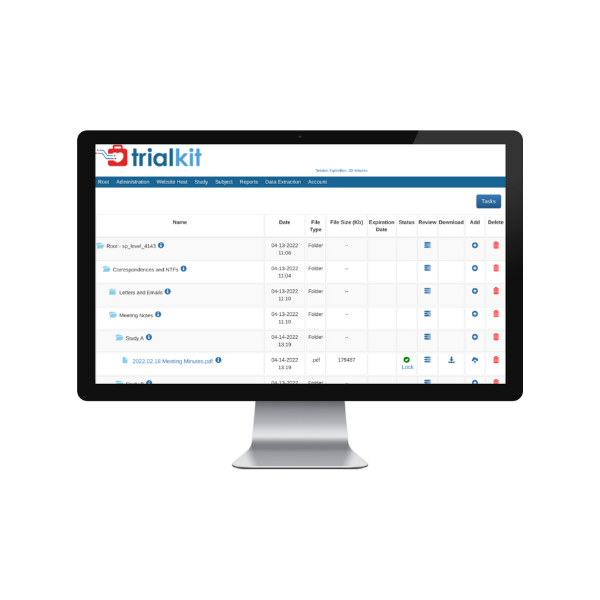

TrialKit eTMF consolidates mission-critical documents in a central location for easy access for faster TMF delivery and study execution.
Manage your clinical trial documents in real-time for truly remote collaboration between sponsor, site, and CRO
What Is an Electronic Trial Master File (eTMF) in Clinical Research?
An Electronic Trial Master File (eTMF) is a digital system used to collect, manage, and store essential documents throughout the lifecycle of a clinical trial. It replaces traditional paper-based filing systems with a secure, cloud-based solution that improves efficiency, transparency, and regulatory compliance.
In clinical research, an eTMF serves as the central hub for maintaining documentation that proves the trial was conducted in accordance with regulatory requirements. From protocols and investigator brochures to site communications and approvals, an eTMF ensures that every document is version-controlled, audit-ready, and accessible when needed.
With TrialKit eTMF, research teams gain real-time access to critical documents, automated workflows for submission and approval, and built-in compliance with global standards like FDA 21 CFR Part 11—all within a modern, user-friendly interface.
How TrialKit eTMF Works
TrialKit eTMF is an intuitive, cloud-based solution that supports the entire lifecycle of clinical trial documentation—from initial uploads to final archiving. Designed to simplify regulatory workflows and reduce administrative burden, TrialKit empowers sponsors, CROs, and sites to stay aligned and audit-ready at every phase.
Real-Time Access for Study Teams, Sponsors, and CROs
Cloud-based infrastructure and native mobile apps enable real-time access from anywhere, allowing decentralized teams to collaborate efficiently. Monitor document status, approvals, and version history with full transparency.
Intelligent Workflows and Automated Filing
TrialKit eTMF automates key tasks like document routing, version control, and approval tracking. This minimizes manual effort and reduces the risk of missing or misplaced documentation.
Compliance-Built Architecture for Global Regulatory Standards
Built to support FDA 21 CFR Part 11, GDPR, and other international frameworks, TrialKit eTMF features robust audit trails, permission-based access, and electronic signature capture to meet even the most stringent regulatory requirements.
TrialKit eTMF for Clinical Trial Efficiency and Oversight
TrialKit’s eTMF simplifies day-to-day document management and helps accelerate trial timelines. By automating routine workflows, reducing reliance on email, and eliminating the inefficiencies of paper-based filing, your team can focus more on moving the study forward.
With real-time document visibility and a centralized platform, site monitoring becomes faster, inspections become less stressful, and cross-team collaboration improves. TrialKit eTMF also integrates seamlessly with the broader TrialKit platform, including tools like EDC, eConsent, and ePRO, giving you a unified system that supports clinical trial operations from start to finish.
How TrialKit’s eTMF Software Tranforms Clinical Document Management
Move away from error-prone paper- and spreadsheet-based processes. TrialKit’s eTMF system integrates with TrialKit EDC as well as external vendors in the cloud, ensuring your documents stay current, accurate, and inspection ready.
Stop sorting through paper and spreadsheets
TrialKit eTMF consolidates your trial documents into one central location. Your documents are easily accessible from anywhere in real-time, making remote collaboration a reality.
Get off the ground in days (not weeks)
TrialKit eTMF comes with a built-in template based on CDISC’s Trial Master File (TMF) Reference Model v. 3.3.1. Combined with the drag-and-drop functionality inherent in all TrialKit products, you can implement TrialKit eTMF in days, not weeks.
Integrate with multiple vendors
TrialKit eTMF pairs nicely with the TrialKit platform. As a standalone offering with RESTful API, it plays well with other data collection and management systems and applications. Use it for multiple studies and with multiple vendors.
Designate custom workflows
Design workflows to suit your study. Create custom folder structures, change folder and file properties, and maintain regulatory data in a central location.
Secure access among sponsors and sites
Role-based permissions allow administrators to designate who can view, edit, and upload/download eTMF documents. You can also allow certain site staff and study team members to access, edit, and share essential site documents from the same repository.
Speed up trial execution
eTMF nearly eliminates risk of errors caused by misplaced or mismanaged documents. Instead, you get inspection-ready documents stored in a system that grows with your organization.
Get a complete audit trail
To ensure compliance, all transactions and changes in TrialKit eTMF are logged in a verifiable and reportable audit trail, including file versioning information.
Why Leading Sponsors and CROs Choose TrialKit for eTMF
Sponsors and CROs turn to TrialKit eTMF because it’s scalable, easy to implement, and designed for modern studies. Whether you’re running a single-site Phase I study or a global Phase III program, TrialKit adapts to your needs with configurable templates, flexible user roles, and enterprise-grade security.
Scalable and study-ready: Ideal for studies of any size or complexity
Modern, mobile-friendly interface: Intuitive design for faster adoption
Backed by expert support: From onboarding to ongoing optimization, our team is here to help
Part of a unified platform: Streamline operations by combining eTMF with EDC, eConsent, ePRO, and more
TrialKit eTMF isn’t just another filing system. It’s a smarter way to manage compliance and trial oversight.

