Your End-to-End eClinical Platform
Run complex clinical trials and massive registry studies without a huge technology price tag. No coding, no waiting.
Are you tired of spending months on a clinical study build? Frustrated by overpriced technology that underdelivers?
Stop waiting on database migrations, custom coding, and access to your data. Launch your clinical trials and registry studies in days with a purpose-built, end-to-end solution. TrialKit’s drag-and-drop development environment eliminates complicated, time-consuming programming.
With an easy-to-use platform accessible via mobile or web app, busy research teams have more time for what matters—your patients. With the latest mobile and AI features, data collection and management tools, and reporting options all easily accessible, TrialKit powers even the most complex clinical trials—your way.
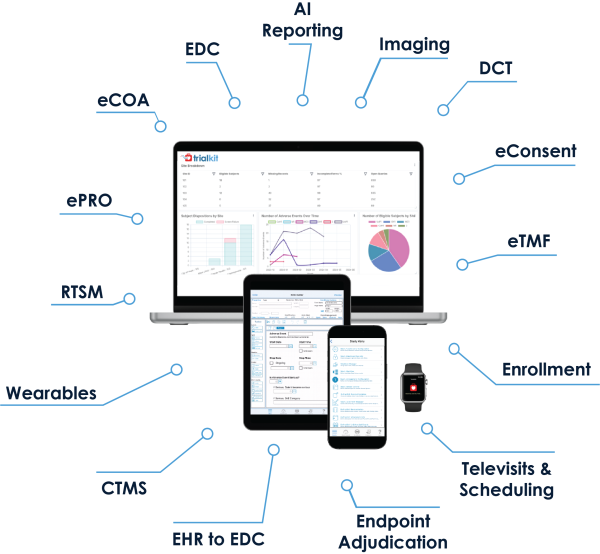
We love the flexibility to modify live studies, reuse CRFs (which hastens development time for each successive study) and create stacked edit checks and conditional actions. The transparent pricing structure removes the high costs associated with launching and running studies.
Clinical Data Manager
We rely on TrialKit to document the collection of dried blood samples and upload data from users’ mobile devices to secure storage in the cloud. Collaborating with the experienced TrialKit team has been a very cost-effective method to rapidly design and test a capable and polished product.
CEO
Why Trust Your Studies to TrialKit?
TrialKit supports all study models, therapeutic areas, and levels of complexity. From preclinical research, through Phase 1 – 3, all the way to post-market evaluation and Phase 4 registry studies, TrialKit offers cost-effective modules for efficient data collection and study management.
Get a Truly End-to-End Solution
- Modules include EDC, eSource, eCOA/ePRO, eConsent, eTMF, RTSM, Medical Coding, Virtual Visits, PACS, and AI Reporting/Analytics. EHR-to-EDC capabilities accelerate registry and Phase 4 studies
- All modules operate on both mobile and web applications so you can run your studies from anywhere on any device: smartphone, tablet, or desktop computer
- Add or remove modules according to your study needs
- Make mid-study changes on the fly with no downtime
- TrialKit’s low-code/no-code environment enables truly DIY study builds
Protect Your Data

- Define user roles and access to data for site personnel, sponsors, CROs, study teams, and study participants via over 250 configurable role-based options
- Software and data center comply with SOC Type 2, Privacy Shield EU-US, and HiTrust
- TrialKit is compatible with all modern web browsers and mobile operating systems
- Disaster recovery, data backups, redundancy, fail-over features guarantee 99.7% uptime
- Data corruption protection and enhanced data verification via blockchain APIs
Maintain Compliance

- Software and data center comply with 21 CFR Part 11 guidelines, GDPR, HIPAA, EMEA Annex 11, ICH Q9, ISO 9001:2015, ISO/IEC 27001:2013
- ALCOA+ data integrity via blockchain APIs
Support Patient- and Site-Centricity

- Deploy TrialKit using a BYOD approach, available as a downloadable mobile app for iOS, Android, and Mac devices, to eliminate the costly need to provision devices to sites and study participants
- Use biometric technology (i.e., Touch ID and Face ID) to login to TrialKit through compatible smart devices and enable two-factor authentication
- Multilingual, end-user-defined interface (currently available in 16 different languages)
- Single sign-on (SSO) enables access to an unlimited number of studies in one place
- Automatically upload images, video, and audio recordings directly from a smartphone or tablet directly into TrialKit
Worry-Free Infrastructure & Hosting
- Centralized, cloud-based platform hosted by Amazon Web Services (AWS) for compliant, secure, and reliable hosting
- Data centers are located strategically worldwide for speed and security. When data privacy laws dictate that you must maintain data in a certain geographic region, we’ve got you covered
- Ability to synchronize multiple clouds in accordance with the needs of a specific study or program of studies across geographic regions
- Run multiple clouds with single sign-on (SSO)
Custom Branding and Private Clouds

- White/private label option: CDS will set up, configure, and deploy a branded version of the TrialKit mobile app (i.e., white-labeled) that you can offer as your own downloadable app from the App Store and Google Play
- Private cloud option for when you need a customized, dedicated hosting environment within the CDS hosting ecosystem within AWS
Unlimited Integration
- Fully documented, modern RESTful API enables the integration of data to/from any external system or database (i.e., EDC, eTMF, CTMS, IRT, or imaging system)
- Integrate with third party data sources to pull data from wearable devices, electronic health records and any Bluetooth-enabled device (implants, sensors, etc.)
- Over 1,000 unique calls available with TrialKit’s API
Support Available 24/7
- 24/7 help desk support available via email and online request
- Live technical support available during business hours (8 a.m. EST-5 p.m. EST)
- Live, hands-on study setup and configuration support during the first 90 days of enablement for any new implementation
- Access an extensive library of online, self-service training tools, including a knowledge base with AI-powered search, videos, and training wizards
Get Up to Speed in Days

- TrialKit’s drag-and-drop environment allows you to get started in less than one day and get TrialKit Administrator-certified within a week. Spend less time learning the platform and more time on your study
- Re-use existing data, validations, configurations, and forms to save hours of time
- Complete end-user testing in minutes—not days—with TrialKit’s auto-validation capabilities. Combined with role-based testing, you get a system designed for quality.
- Ability to operate in 5G and 6G platforms for the decentralized clinical trials of tomorrow

