Although approach differs slightly amongst organizations in clinical research, everyone agrees on this: it’s time for the industry to become more agile. Too many life-saving treatments and medical advancements are at stake to remain attached to the traditional ways of data collection. Over the past ten years, we’ve seen things slowly going the way of virtual clinical trials. Pharma World Magazine provides a nice definition of virtual clinical trials: they’re trials that “take full advantage of technology (apps, monitoring devices, etc.) and inclusion platforms (recruitment, informed consent, counselling, measurement of endpoints and any adverse reactions) to allow the patient to be homebased at every stage of clinical trial.”
The benefits of virtual trials are numerous, and we’ll cover many of them in this blog post. TrialKit is lowering the barrier to entry for everyone involved in clinical trials – sponsors, sites, and patients alike. Let’s walk through the different ways TrialKit can make it achievable for you and your team to conduct virtual studies.
Facilitate study participation and improve retention rates
Active participation from patients is the lifeblood of clinical trials. Likewise, high dropout rates and inadequate amounts of data can cause a study to fail. For this reason, patient centricity has become an imperative element of trial design. TrialKit, available as a native mobile app for iOS and Android, was developed with both research professionals and patients in mind. The app serves as a technology tool for researchers to build, deploy, and manage studies. It also provides clinical trial participants with an easy-to-use platform to complete forms and surveys ePRO-style. To make TrialKit truly accessible to all, it can be downloaded on the App Store or on Google Play.
Decreasing the amount of study site visits required by patients makes it much more convenient to partake in a trial. Additionally, the remote nature of virtual trials means researchers may now include people who experienced difficulties travelling to sites in the past. Now, those who lacked transportation or dealt with geographical barriers have the ability to participate in studies that were previously inaccessible.
There’s another factor at play when it comes to patient-centered virtual trials: user-friendliness. Research professionals have ultimate flexibility in eCRF design with TrialKit’s drag-and-drop form builder. Creating forms that are easy for patients to fill out and submit furthers the convenience of virtual trials. And, completing forms in TrialKit is as instinctive as using other apps mobile device owners access on a daily basis.
The mobile format of CRFs offered by TrialKit is far less restrictive than paper CRFs. Instead of leaving a site with a stack of paper forms to keep track of until their next visit, study participants can access them from any iOS or Android device. Simple fields such as date, multiple choice, and short answer can of course be included on CRFs. However, TrialKit is capable of collecting data from more advanced fields, including photo, video, audio, sketch pad for annotations, and signature for electronic informed consent (eConsent) purposes. Beyond that, barcode scanning may also be used in forms and surveys. As an example, a patient could use TrialKit to scan a barcode attached to a medication they have been prescribed for a study to demonstrate that they have taken it. That product can then be tracked with that patient in the trial supply for the entire study to provide an exact audited history of the drug or device. Removing the burdensome parts of participation – like paper CRFs – and making the process more enjoyable will take patient engagement and retention a long way.
Around the industry, concerns have been raised about patient safety in virtual trials. Patient safety remains a principal focus in the design of TrialKit, and our developers give special attention to the features and functionality that support the virtual aspect of trials. In the study design process, researchers may configure CRFs to trigger real-time notifications in instances of adverse events (AEs), serious adverse events (SAEs), and more based on data submitted by patients. Another part of study configuration allows researchers to set up notifications that inform patients when forms or activities are due, either within a specified time window or in intervals which are dependent on some event. Additionally, the system comes with built-in messaging. This way, study managers or site coordinators are able to connect with patients directly if there is important news to share.
It’s important to note that in order for patients to participate in a virtual trial, they don’t need a smartphone or tablet. eCRFs may also be filled out using any web browser. Enabling patients to participate remotely requires flexibility to allow the use of devices people already own.
Collect more data with less effort
In addition to patient participation and retention, a sufficient amount of high quality data is integral to the success of a study. This is absolutely feasible in a virtual clinical trial when using tools like TrialKit. The platform operates on an open system via RESTful API, which we’ve covered extensively in a past blog post. The open nature of TrialKit gives research teams ultimate flexibility to collect and manage clinical data. It simply means the system has the ability to integrate with any third party systems.
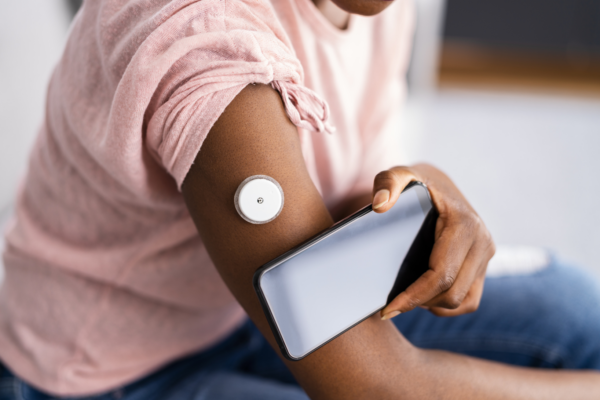
This even includes connectability to devices – Apple Watch, FitBit, or any other wearables – that generate relevant data. Integrations with wearables are sophisticated enough to reduce drastically effort for both research teams and patients. For example, with a patient’s consent, researchers can configure TrialKit to record heart rate from a wearable at predefined intervals.
Similarly, wearables and devices can produce objective data while saving study participants from attending multiple site visits. TrialKit harnesses the power of core sensor technology in iOS devices to allow for activity tests. Patients may carry out range-of-motion tests, step tests, and more at home or any preferred location. With these sorts of passive tests that can be performed on the go, patients can provide higher volumes of data around a specific treatment outcome, all at a much lower cost than it would otherwise take to gather even a fraction of that same data.
Shorten study cycles
A handful of characteristics contribute to an overall shorter study duration when it comes to virtual trials. First, patient reported outcomes (PROs) submitted electronically, also known as ePRO, become available to research teams much quicker than their paper counterparts. Forms and surveys submitted on TrialKit hit in the database in near-real-time. Notifications can be created to inform researchers immediately when this happens, giving them more time for analysis. Next, submitting data electronically lessens transcription errors. Although there is still room for error when inputting data on a phone, tablet, or computer, built-in edit checks in TrialKit guide users to correct mistakes.
Future implications of virtual clinical trials
The Silent Generation and Baby Boomer populations are tapering off slowly. As time goes on, older people are becoming more tech savvy and more comfortable with using mobile devices. And with support from the Food and Drug Administration (FDA) in the 21st Century Cures Act surrounding PROs, the healthcare industry is inching closer to the modernization it desperately needs. In just a couple decades, mobile device based data collection will be the standard.
At Crucial Data Solutions, we envision the perspective on virtual trials to shift in the years to come as big data enters the scene. Someday, the massive amount of existing structured and unstructured clinical data will fuel machine learning technology in helping us conduct research in ways not yet done before.
Of course, the virtual format isn’t realistic for all trials when scheduled site visits are necessary. Many research teams have found a hybrid model to be an answer to this, incorporating both site visits and remote monitoring in their processes. TrialKit currently supports a number hybrid trials, and we look forward to many more as this methodology gains momentum. Get in touch with us today to talk all things virtual.