
-

Making Data Exchange Feel Easy – Simplifying the Data Journey from EHR to EDC
Demystifying and providing a solution for EHR to EDC interoperability has been a mission for CDS for the past four years. In this blog post, we break down various interoperability challenges and how the architecture of your eClinical software can be a game-changer.
-

It’s Your Data: Open API Gives You Better Access and the Freedom to Build and Manage Your Studies, Your Way
Unlike some providers in the eClinical space, at CDS we do not hold your data hostage when you conduct your studies in TrialKit. In fact, TrialKit is driven by an open API that gives users true freedom and access to their data – whenever they need it. Read on to learn how we do this…
-

Low-Code or No-Code Software to Manage Clinical Trials – Tools to Build Your Studies, Quickly
The software space is experiencing a movement toward low-code/no-code development practices. But what exactly does this mean, and how does it apply to clinical trial management? This blog post introduces you to the low-code/no-code approach and why it might be a good fit for you.
-
Crucial Data Solutions Introduces Cloud Computing to its eClinical Technology
Research professionals using TrialKit to conduct clinical trials now have the option to house data on a private cloud server located anywhere in the world.
-

What are the Benefits of Private Cloud Storage in Clinical Research?
Private clouds are quickly becoming an alternative to typical cloud storage for clinical trial data. If you’re wondering what sets the private cloud apart, this blog post breaks down its various benefits.
-
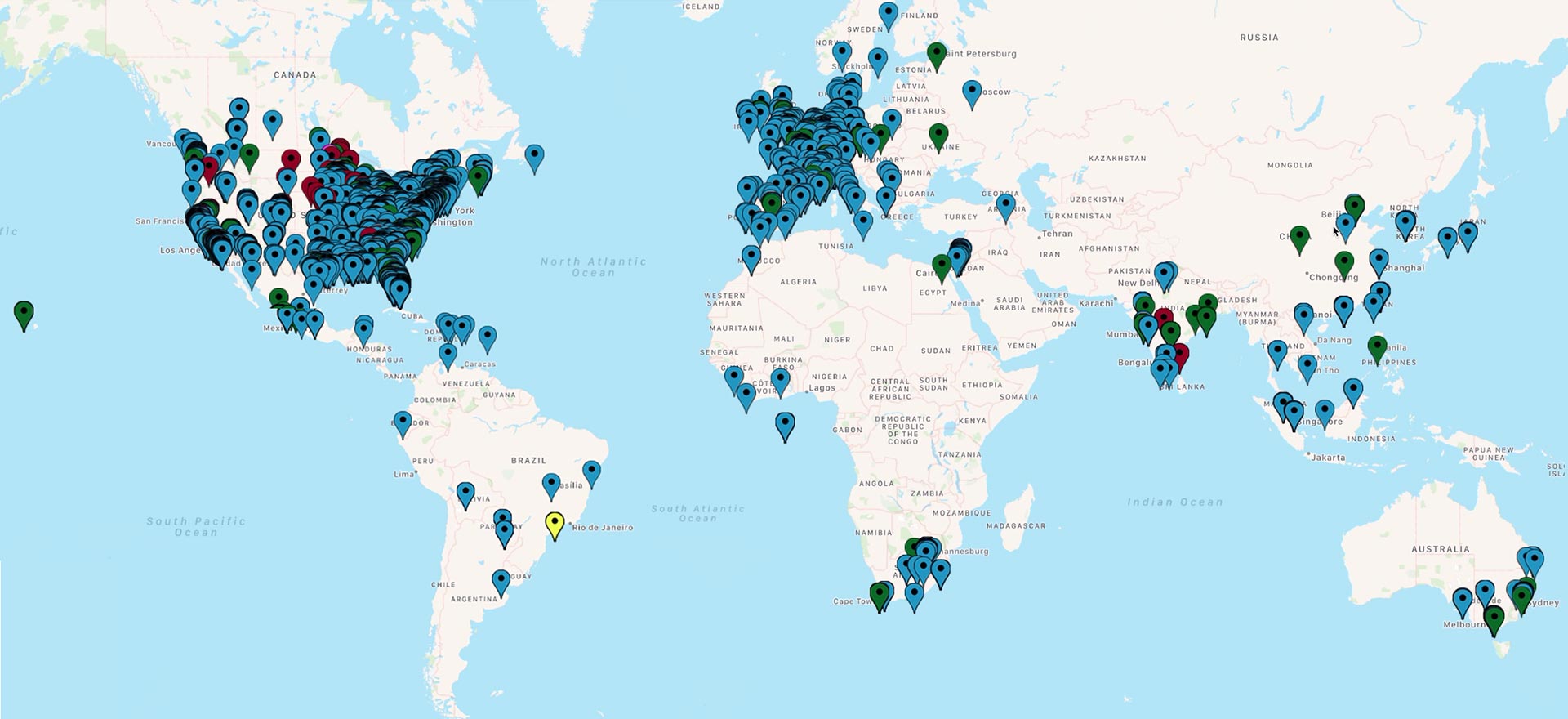
Crucial Data Solutions’ eClinical Platform Reaches 10,000 Users
The first quarter of 2019 has brought about a meaningful milestone for Crucial Data Solutions. Users of TrialKit’s web-based eClinical platform and native mobile app have surpassed the 10,000 mark.
-
Open APIs and Webhooks: The Key to Interoperability in Clinical Research Technology
In this post, we’ll delve into what APIs and Webhooks are, how they function, how they’re being used as an engine for interoperability today.
-
Clinical Trial Database Storage Requirements: What to Consider
What should research teams take into consideration when evaluating a data collection and study management solution’s storage capabilities? In this post, we take a look at different elements of database storage and how they apply to clinical trials.