Remote Patient Monitoring and Wearable Integrations
Collect quality data from your participants remotely to launch smooth-running decentralized clinical trials and digital health studies.

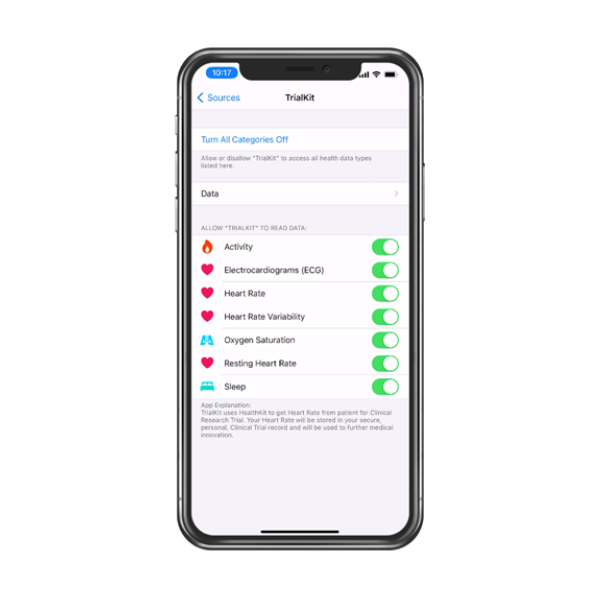
Connect wearables through a single unified platform
TrialKit supports Apple Health, Google Health Connect, and Fitbit data sources. Any device or sensor that sends data to these ecosystems can be used in a study, including popular fitness trackers, smartwatches, connected health devices, and specialty sensors. Integrations with additional devices can also be accommodated upon request.

Capture a Wide Range of Health, Activity, and Sensor Data
TrialKit can collect many types of participant-generated data from wearables and mobile-connected devices. These data points flow into study records to support digital endpoints, remote assessments, symptom tracking, and continuous monitoring. Common data types include:
- Additional metrics depending on the device and protocol
- Steps and activity minutes
- Heart rate and heart-rate variability
- Sleep duration and sleep stages
- SpO2
- Temperature
- Mobility and gait metrics
- Passive background activity
- Pain entries captured through the TrialKit app
- Raw sensor streams when supported by the device
How Wearable Data Flows Into TrialKit
Participants use the TrialKit mobile app to authorize data sharing from their devices. TrialKit retrieves data from Apple Health, Google Health Connect, or Fitbit APIs and routes it into visit schedules, CRFs, and study dashboards. Sites, monitors, and data teams can review those values in real time. The process is simple for participants and sites:
Participant downloads the TrialKit app
Participant connects their preferred device or data source
TrialKit retrieves health and activity data via API
Data appears inside the study database in structured fields
Study teams view data through CRFs, reports, and monitoring tools
Why Choose TrialKit for My
Decentralized Clinical Trial?
Integrating data from multiple data sources creates data management nightmares. Keep your team sleeping peacefully by using a data capture and management platform built with mobile in mind.
Multiple sources, one repository
TrialKit consolidates wearable data in the same secure environment as your EDC, ePRO, and visit data. Study teams access a complete, centralized repository without managing separate systems.
High-volume data handling
TrialKit can ingest both common wearable metrics and high-frequency sensor data when supported by the device. This enables digital endpoints and continuous insights needed for modern studies.
Configurable study-level controls
Study builders choose which metrics to collect, how often data should sync, and how values appear in the database. All configuration is handled through familiar TrialKit tools.
Real-time visibility for sites and monitors
Synced values appear inside CRFs, dashboards, and reports. Sites and monitors can quickly review trends, patient progress, and safety indicators.
Secure and compliant data governance
Role-based permissions and audit processes protect participant data. TrialKit manages access based on study roles and regulatory requirements.
Unified with every TrialKit module
Wearable data seamlessly aligns with ePRO, remote visits, and EDC workflows. Teams work from one platform instead of stitching together multiple vendors.

Use Cases Across Therapeutic Areas
Wearables support continuous, real-world insights across many types of clinical research. TrialKit can be configured for simple passive tracking or advanced digital endpoints. Popular use cases include:
- Chronic pain studies
- Neurological and movement disorders
- Cardiometabolic and diabetes studies
- Respiratory conditions
- Sleep research
- Remote safety monitoring
- Digital biomarker development
- Hybrid and decentralized studies
We rely on TrialKit to document the collection of dried blood samples and upload data from users’ mobile devices to secure storage in the cloud. Collaborating with the experienced TrialKit team has been a very cost-effective method to rapidly design and test a capable and polished product.
Dr. Leigh Anderson, CEO of SISCAPA Assay Technologies



