PACS Imaging Software for Researchers
Access, interact with, and even adjudicate clinical trial imaging from the same platform where you manage all other study data.
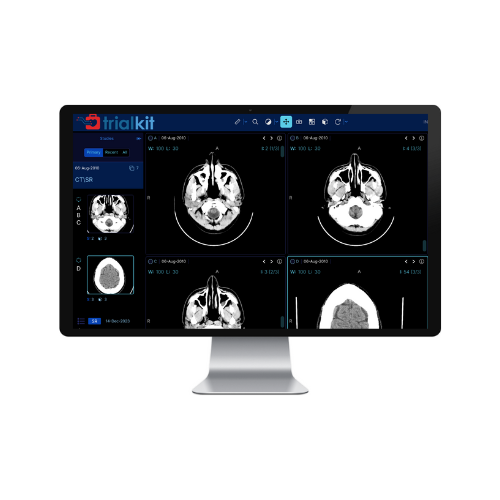
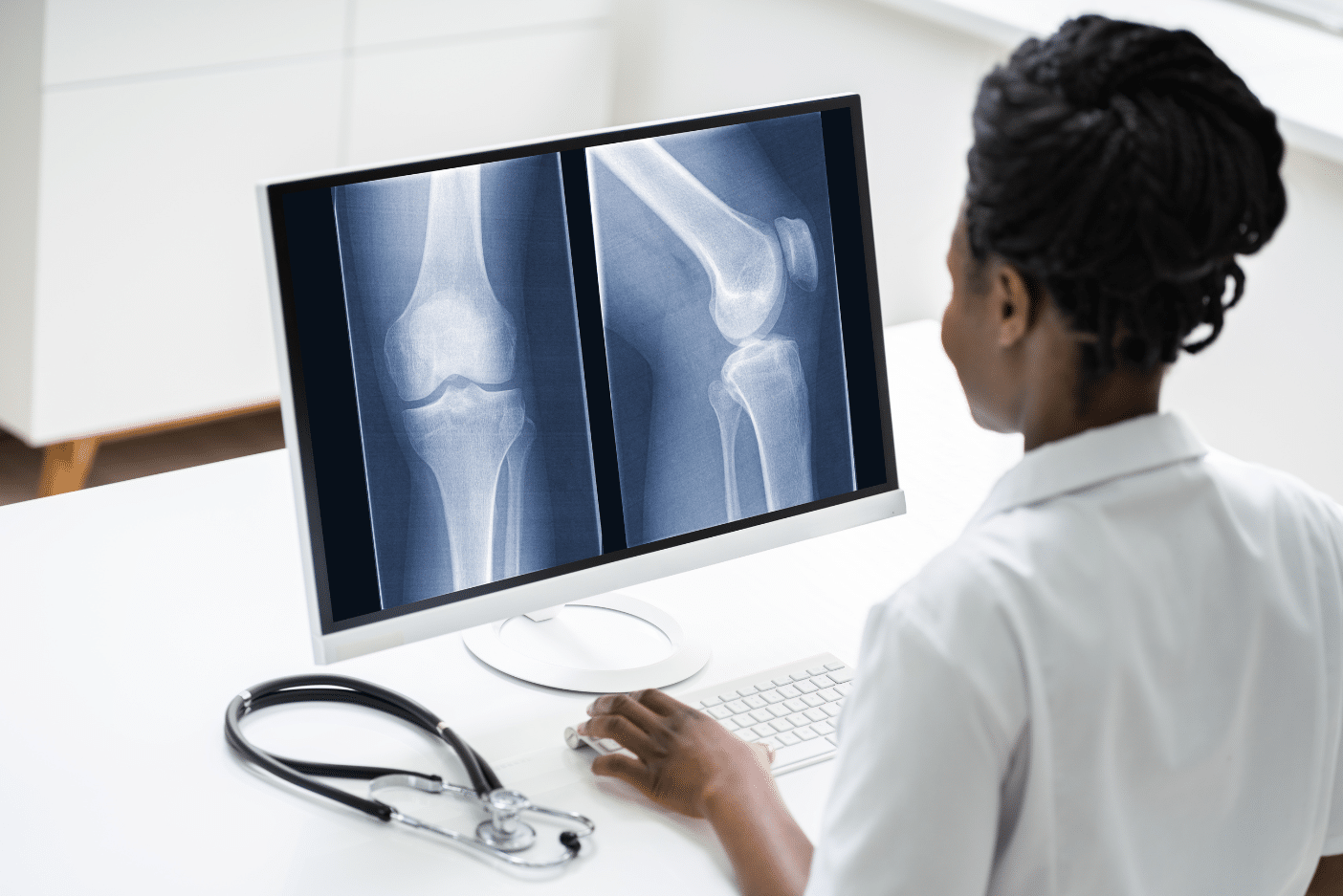
A Clinical Trial Imaging Solution for all Your Research Needs
TrialKit PACS supports a wide range of imaging modalities commonly used in clinical research, making it easy to collect, store, and review medical images across multiple study types and therapeutic areas. Whether you’re conducting an oncology trial, a neurology study, or imaging-based endpoint research, TrialKit’s flexible imaging capabilities can meet your needs. Supported imaging formats include:
X-rays for assessing bone structure, fractures, and orthopedic conditions
Magnetic Resonance Imaging (MRI) for high-resolution views of soft tissue, brain activity, and tumors
Computed Tomography (CT) scans for cross-sectional imaging and volumetric analysis
Ultrasounds for real-time imaging in prenatal, cardiovascular, and organ studies
PET scans for metabolic activity monitoring in oncology and neurology research
DICOM and non-DICOM formats to ensure compatibility with virtually any imaging device or vendor system
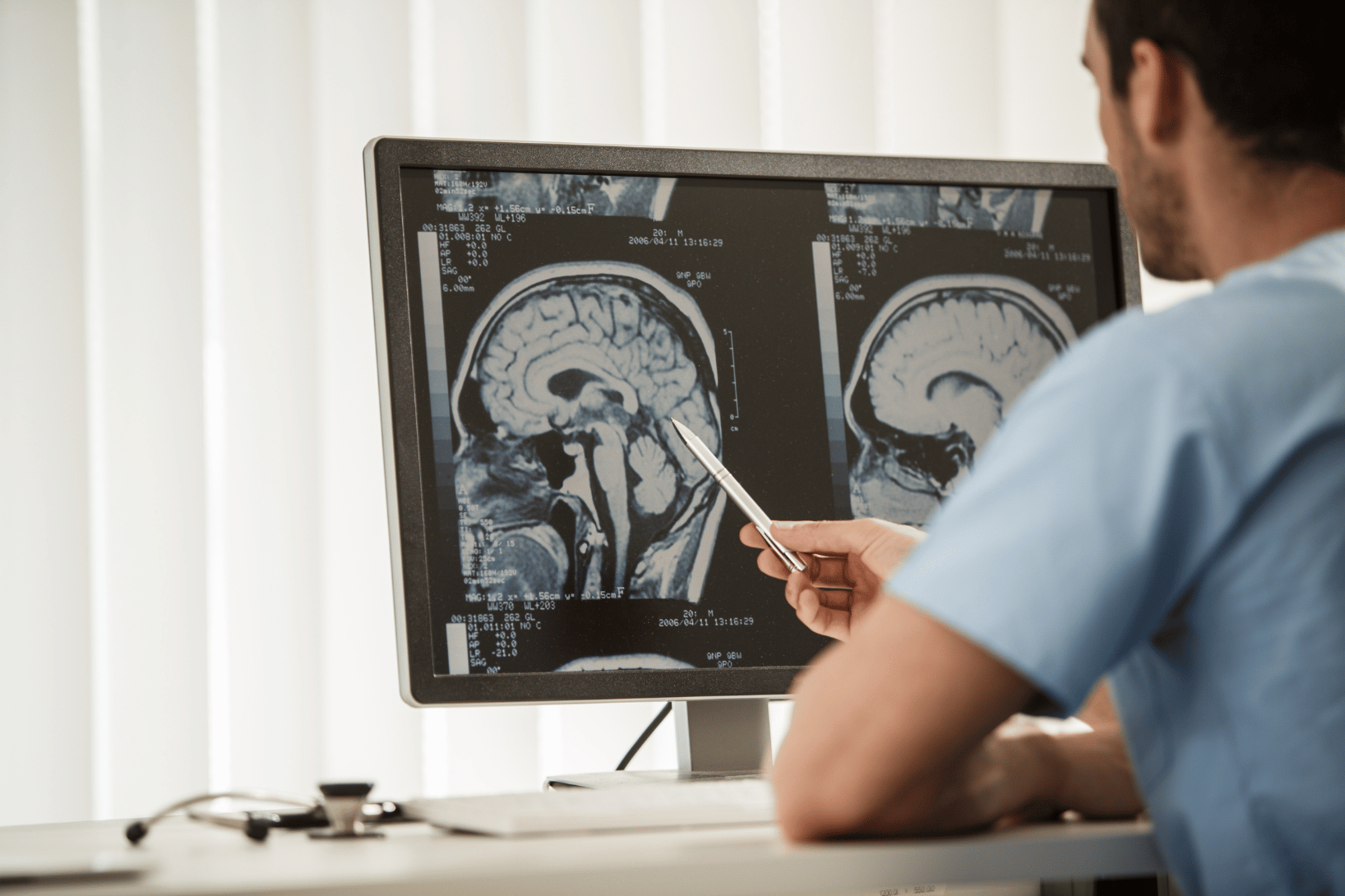
What is PACS?
A picture archiving and communication system (PACS) is a medical imaging technology used to securely store, retrieve, manage, and share digital images such as X-rays, MRIs, CT scans, and ultrasounds. Traditionally used in radiology departments, PACS has become an essential tool in clinical research, helping sponsors and sites streamline imaging workflows across decentralized or multi-site studies.
In the context of clinical trials, PACS serves as a centralized solution for organizing imaging data, reducing manual handling, and improving image accessibility for faster, more accurate evaluations. Researchers can standardize imaging protocols, maintain data integrity, and ensure regulatory compliance while supporting real-time collaboration and remote monitoring.
With TrialKit PACS, medical images are no longer siloed or stored across disconnected systems. Instead, they become part of a unified research environment where imaging data is directly linked to clinical outcomes, making analysis faster, smarter, and more complete.
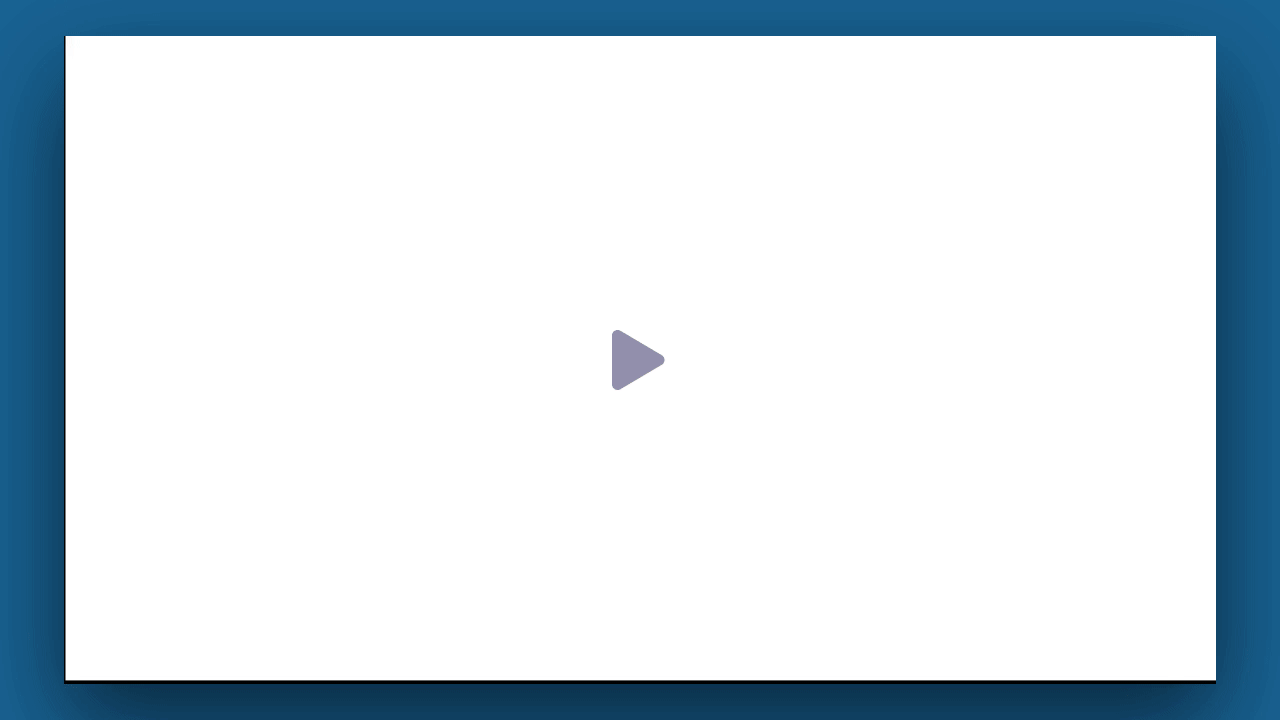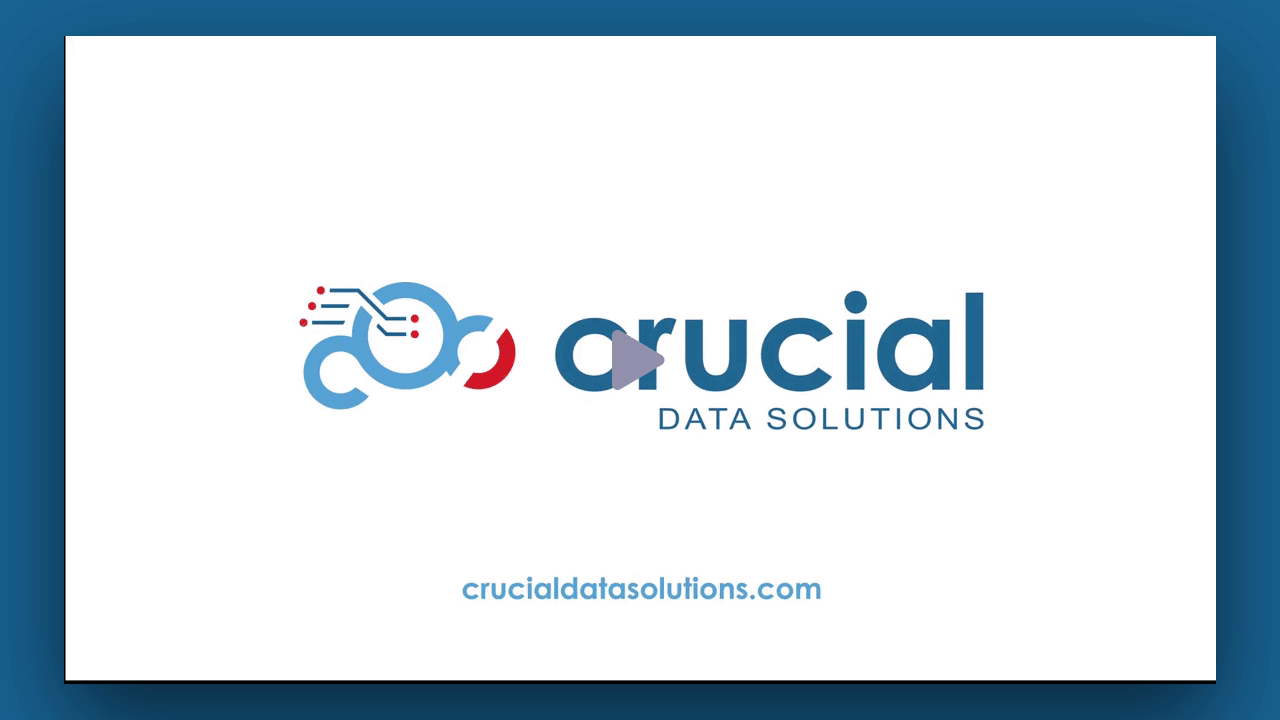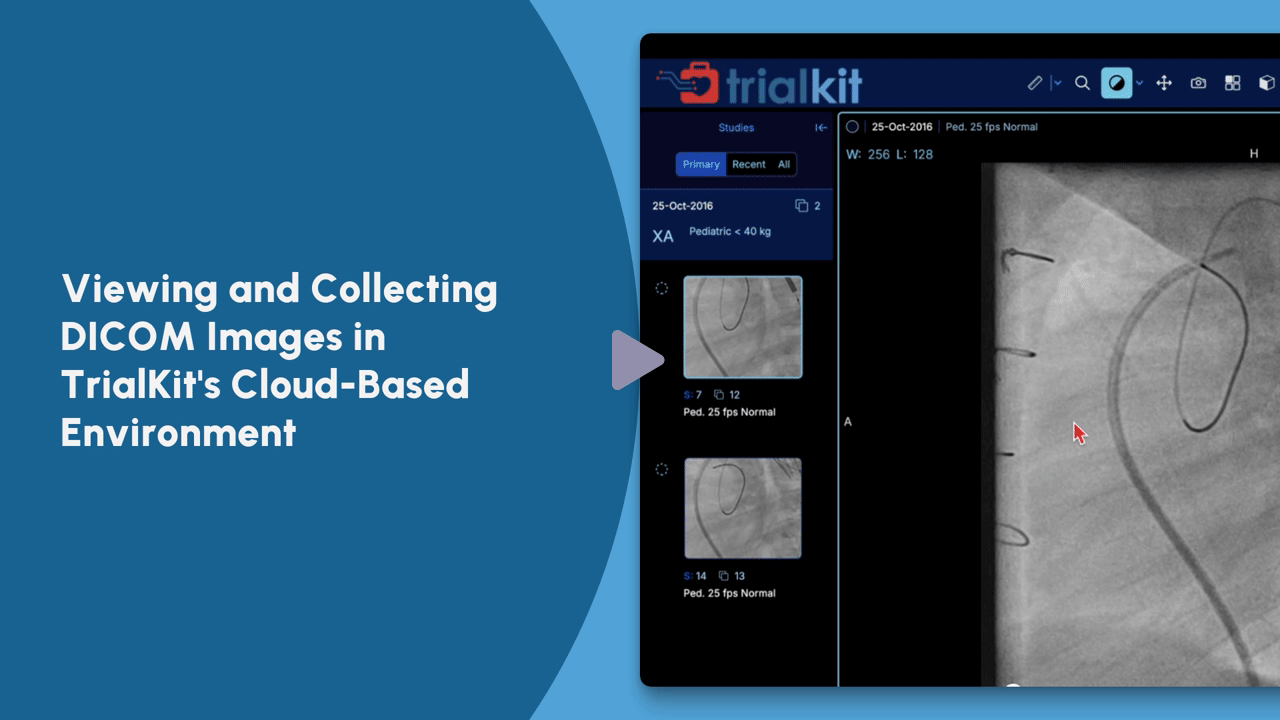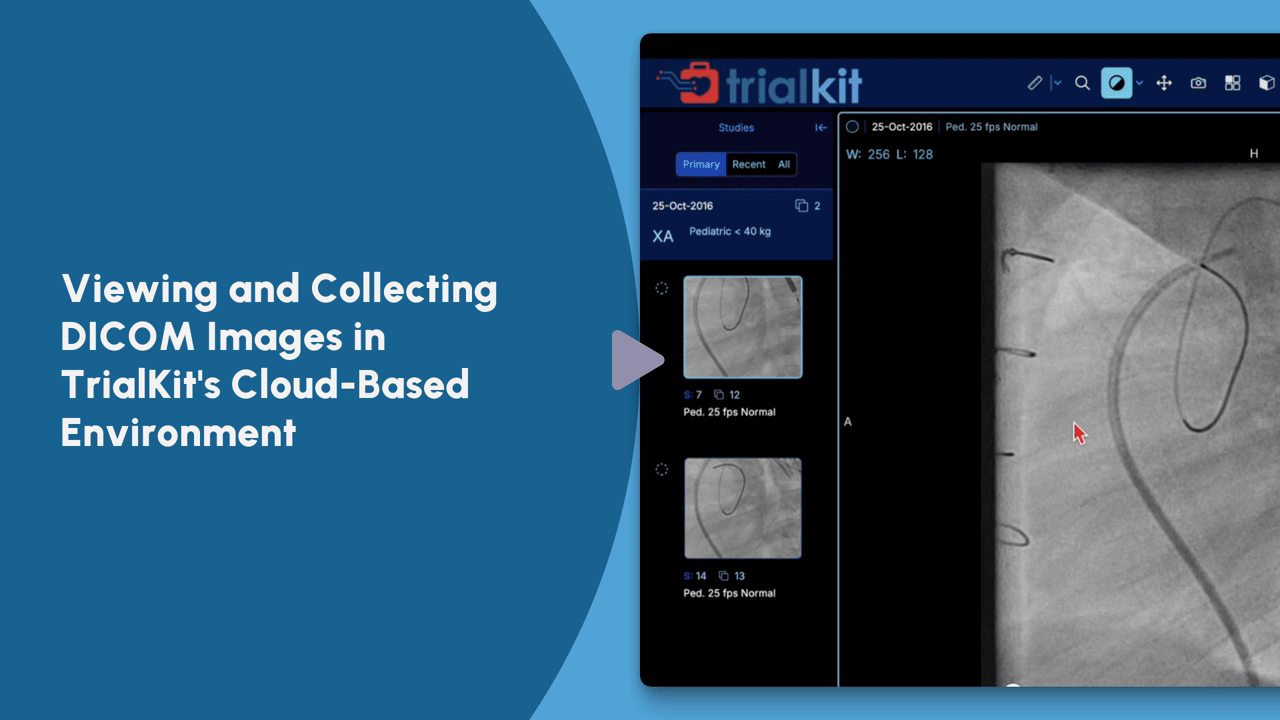
See TrialKit PACS in Action
View this on-demand tutorial for an introduction to TrialKit’s capabilities for storing, retrieving, managing, and sharing imaging data.
Why Choose TrialKit PACS for Your Clinical Trials?
Real-time access and evaluation of DICOM imaging
Researchers can instantly access and evaluate medical images, enabling faster, informed decisions that protect patient safety and data quality.
Robust image interaction
TrialKit’s DICOM viewer is equipped with a range of tools for in-depth image analysis. Researchers can calibrate, zoom, pan, and adjust image view settings, providing a detailed examination of clinical trial imaging.
Anonymize, annotate and collaborate
Images can be quickly anonymized to prevent sharing of sensitive data. Users can annotate images and collaborate with other viewers in real-time, sharing insights, confirming findings, and identifying discrepancies.
Easily integrate imaging into your data analytics
The integration of images into TrialKit’s analytic capabilities allows for a more holistic analysis of trial data. Researchers can easily correlate imaging data with other trial metrics all within the TrialKit platform, allowing them to reach deeper study insights more quickly than ever.
Customizable dashboard reporting
Configure and generate customizable dashboard reports that include imaging data alongside other clinical trial metrics. This feature provides a comprehensive overview of trial progress and outcomes, essential for stakeholder reporting.
Secure and compliant cloud storage
TrialKit ensures the highest standards of data integrity and compliance. Our platform adheres to global regulatory requirements, guaranteeing that the storage and handling of medical images meet stringent security protocols.
Audit trails for enhanced accountability
Every interaction with images within TrialKit is meticulously logged. These audit trails enhance accountability and transparency, critical for regulatory compliance and data integrity.

Unified Imaging and Clinical Data Management
TrialKit PACS is fully integrated into the TrialKit ecosystem, giving researchers a powerful, unified platform to manage both clinical and imaging data in one place. This seamless integration eliminates data silos, accelerates image review timelines, and reduces the complexity of working across multiple systems.
By combining PACS functionality with TrialKit’s other tools, sponsors and CROs gain complete visibility into both imaging endpoints and other study data, accessible through a single login. Imaging files can be mapped directly to subjects, visits, and case report forms, making it easy to associate scans with specific timepoints or outcomes
No matter the scale of your study, from single-site to global multi-center trials, TrialKit PACS provides intuitive access to imaging data on both desktop and mobile devices.