eSource & Direct Data Capture for Clinical Trials
Gain confidence in your clinical research data with direct data capture (DDC).

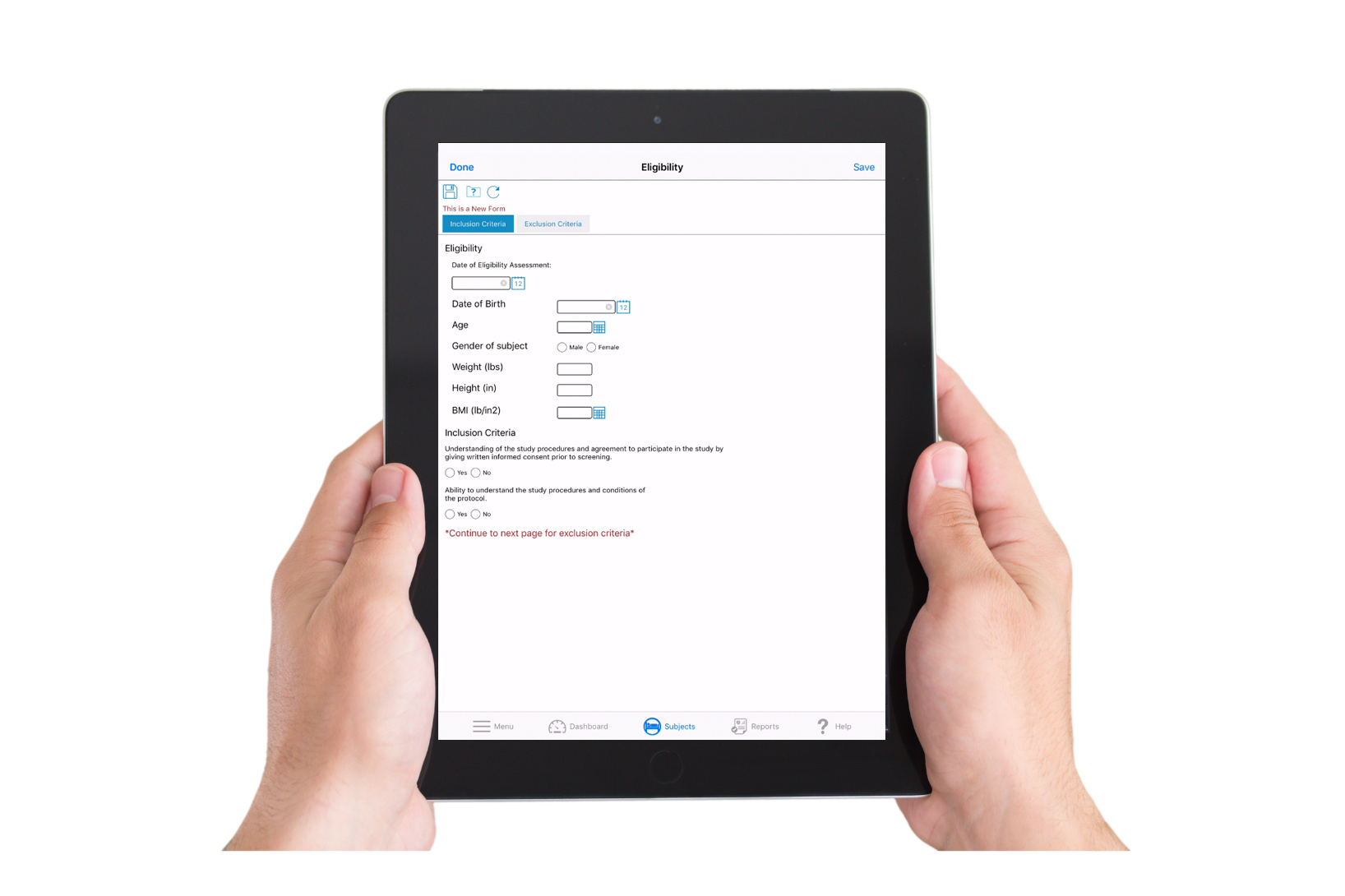

What Is eSource / Direct Data Capture?
eSource refers to clinical information that is recorded directly in digital format at the point of capture, eliminating the need for paper records or later manual transcription. Direct Data Capture (DDC) is one form of eSource—data entered directly into an electronic system (for example, into eCRFs or other site interfaces) rather than first on paper.
By capturing source data electronically and contemporaneously, eSource and DDC reduce transcription errors, streamline data validation, and enable cleaner, audit-ready data from the start.

eSource vs. EDC: Understanding the Relationship
While eSource and EDC (electronic data capture) are often used interchangeably, they represent distinct but complementary concepts:
- eSource / DDC is about capturing the original data electronically at the source (at the clinic, via device, or directly into eCRFs) with no need for paper or transcription.
- EDC is the system (or database) that aggregates, organizes, validates, and stores data from all sites/trials. In modern systems, EDC platforms often support eSource workflows by enabling direct data entry within the same system.
Because of this overlap, many advanced EDC solutions like TrialKit now include native eSource / DDC capabilities, so you get source capture and centralized data management in one platform.
Challenges in eSource / Direct Data Capture Adoption
Even though eSource offers strong benefits, successful implementation requires addressing several challenges:
Site Readiness and Workflow Change
Some sites may lack digital infrastructure or feel resistant to changing from paper-based workflows.
Integration with Legacy Systems
Aligning eSource tools with EHRs, lab systems, imaging systems, and other legacy platforms can be complex.

Data Quality and Completeness
eSource tools must ensure necessary fields, logic checks, and data validations to prevent missing or inconsistent entries.

Regulatory and Validation Burden
Systems must be fully validated, with audit trails, versioning, and compliance controls.
Modern platforms like TrialKit that combine eSource with EDC, built for flexibility and site-friendly workflows, help mitigate many of these challenges.
How TrialKit’s eSource & DDC Capabilities Work
TrialKit offers an integrated eSource / direct data capture module within the broader TrialKit eClinical environment. Here’s how it works:
Point-of-care data entry
Sites can enter clinical observations, assessments, vitals, eCRF fields, and device-generated data directly into TrialKit in real time.
Built-in validations and logic
Edit checks and data rules enforce data integrity at entry.
Unified platform
eSource data flows into the same database used for EDC, ePRO, imaging, and analyses, reducing silos.
Audit trails and versioning
Every entry, modification, and query is logged to maintain traceability.
Remote monitoring and review
Monitors and data managers can access source-level entries to review and flag issues without visiting sites.
Benefits of eSource within TrialKit
- Eliminate double data entry between site source and EDC
- Reduce transcription errors and query volume
- Accelerate data locking and analysis timelines
- Improve data transparency for stakeholders
- Decrease site burden and streamline oversight
- Maintain compliance with global standards