eCOA and ePRO Solutions for Clinical Trials
Planning to launch a patient-centric decentralized clinical trial? Give your clinical trial patients an app that makes it easy to participate—from anywhere at any time.

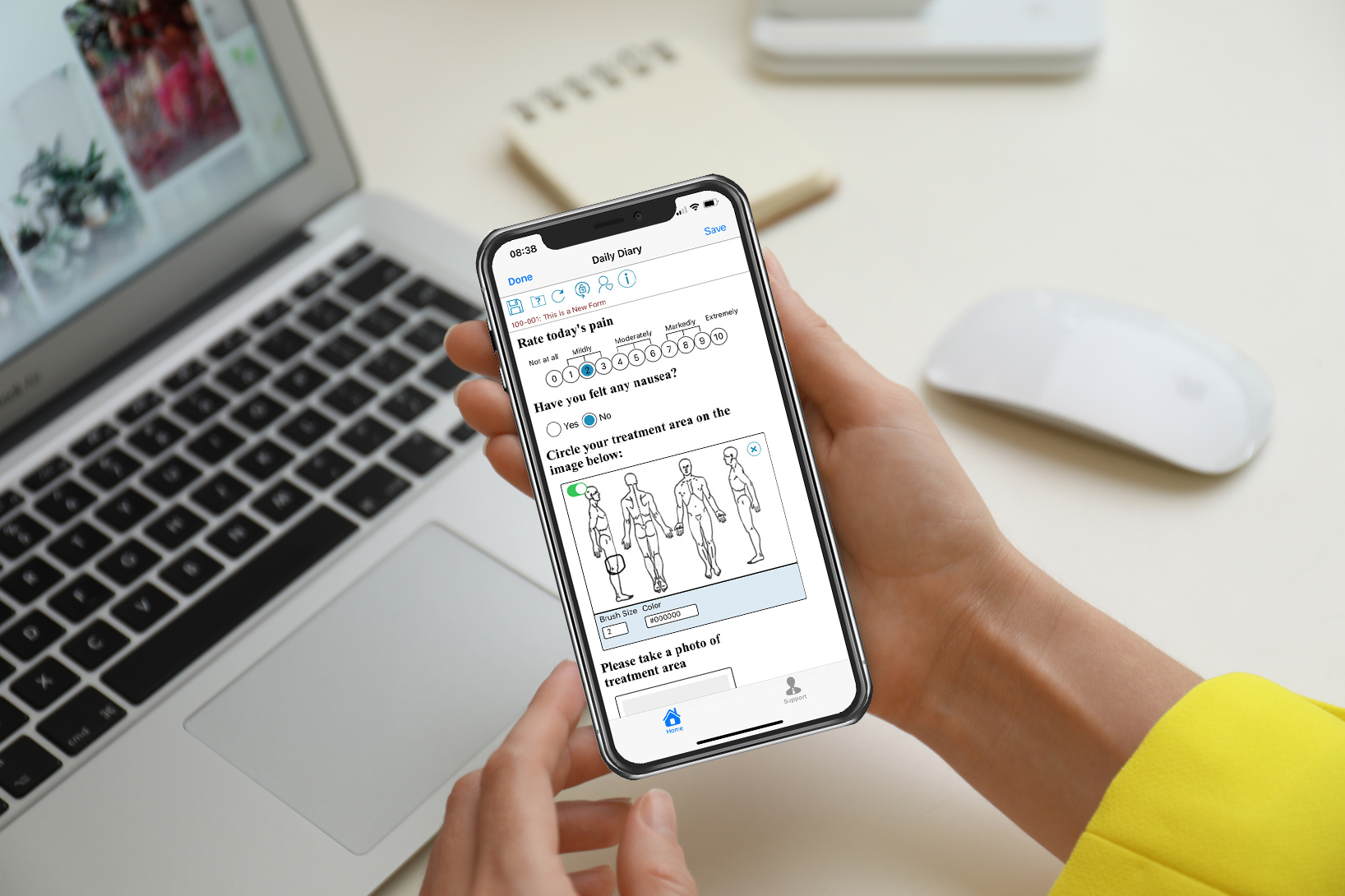

Simplify clinical trial participation with an integrated eCOA and ePRO solution
TrialKit’s electronic clinical outcome assessment (eCOA) and electronic patient-reported outcomes (ePRO) platform integrates with all currently available wearables, mobile operating systems, and web applications. Whether you collect data in the clinic, with provisioned devices, or take a bring-your-own-device (BYOD) approach, you can count on seamless data sharing and efficient study execution.

What Is eCOA in Clinical Trials?
Electronic clinical outcome assessment (eCOA) is the digital collection of clinical outcome data in clinical trials. eCOA captures patient health information reported directly by patients, clinicians, and caregivers using electronic devices such as smartphones, tablets, or computers. By replacing traditional paper-based assessments, eCOA improves data quality, enhances protocol compliance, and supports real-time access to outcomes data. It ensures that data are captured consistently and securely across study sites, whether trials are fully remote, site-based, or hybrid.
There are four primary types of eCOA used in clinical trials:
- Patient-Reported Outcome (PRO or ePRO): Direct reports from the patient on their health status, symptoms, or quality of life, electronically captured through ePRO tools.
- Clinician-Reported Outcome (ClinRO): Assessments completed by clinical investigators based on their observations or interactions with the patient.
- Observer-Reported Outcome (ObsRO): Data provided by a parent, caregiver, or other observer about a patient’s condition, typically used in pediatric or cognitively impaired populations.
- Performance Outcome (PerfO): Results generated through standardized tasks that evaluate a patient’s function, such as a walking test or memory task.

What Is ePRO in Clinical Trials?
Electronic patient-reported outcomes (ePRO) refer to digital self-reports of a patient’s symptoms, treatment experience, and overall quality of life during a clinical trial. As a subset of eCOA, ePRO is focused specifically on the patient’s voice, collected through intuitive mobile apps or web-based platforms.
Using ePRO systems in clinical trials allows for:
- Real-time reporting of health status and adverse events
- Reduced recall bias through scheduled, time-stamped data entries
- Improved patient engagement and adherence via mobile reminders and alerts
ePRO is particularly powerful in patient-centric trials, where understanding the lived experience of treatment is essential to assessing therapeutic value. When integrated into a broader eCOA system like TrialKit, ePRO helps sponsors and CROs capture high-quality, regulatory-compliant data that support more efficient submissions and better clinical outcomes.
Why Choose TrialKit for eCOA and ePRO?
Hassle-free data sharing
TrialKit eCOA/ePRO complement TrialKit EDC and eSource as part of a unified hybrid/decentralized clinical trial solution. Our open API approach enables integration to/from other external systems if you choose.
Empower your sites
Alert site staff when patients experience adverse events and reactions for rapid response. Automated notifications based on real-time data make it happen.
Produce cleaner data
The combination of eCOA/ePRO and electronic case report forms (eCRFs) dramatically reduces the risk of data duplication for less data cleaning and less source data verification (SDV).
Improve patient retention
Patients are more likely to enroll, stay enrolled, and follow the protocol when it’s easy to do so. TrialKit eCOA/ePRO checks that box by allowing BYOD approaches as well as patient-clinician handoffs without patient login.
Real-time data access
Monitor patient adherence and progress in real time. When it’s time for study close-out, your data are ready.
Flexible forms
Access a library of standardized assessments. Configure fields and enable photo/video uploading with a few drag-and-drop moves.
Automate participant reimbursements
Through our integration with nmible, automatically and securely issue reimbursements within the TrialKit platform as participants comply with required data submission activities for studies.
TrialKit eCOA/ePRO: How It Works
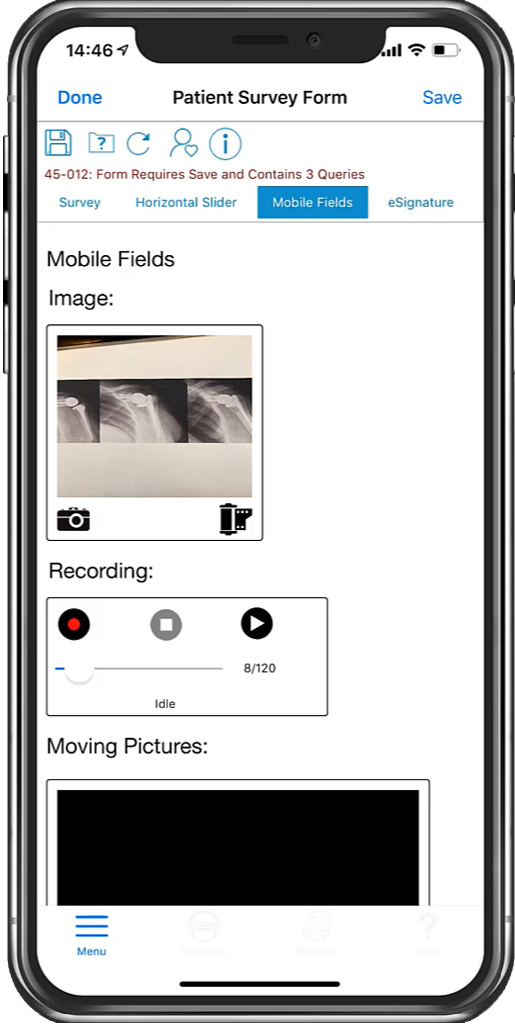
Photo, video, and audio upload directly into the eCRF
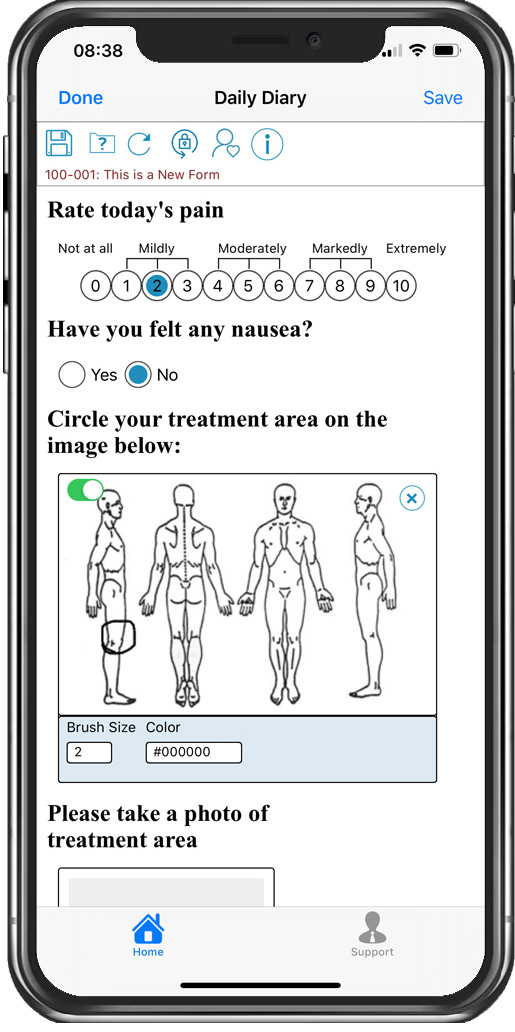
Interactive image capability

Out-of-the-box connectivity with Apple Health, Google Fit, and Samsung Health
to collect real-world data including heart rate, step motion, range of motion, and more.
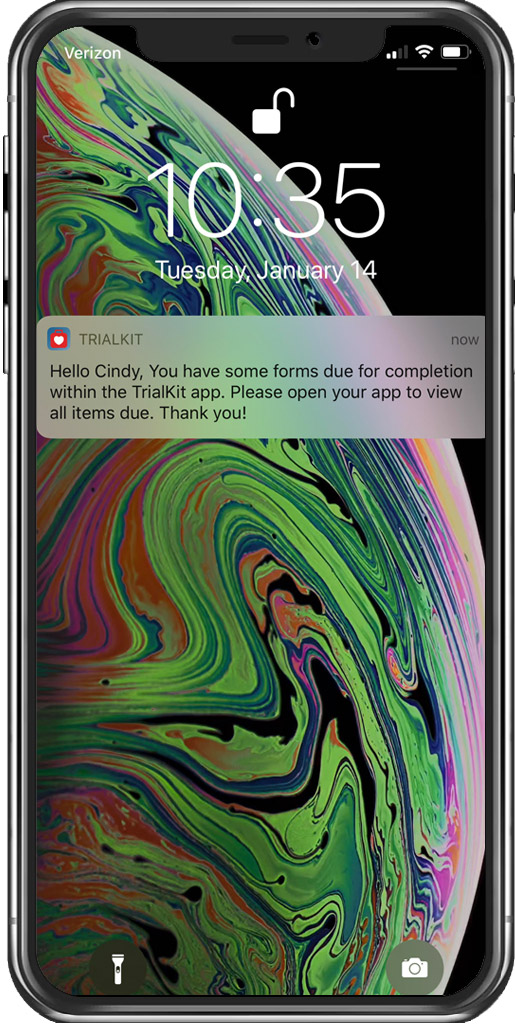
Enforce survey completion
by sending reminders via email, push, and/or SMS notifications that guide study participants directly to their surveys
Benefits of eCOA and ePRO in Clinical Trials
Implementing electronic clinical outcome assessments (eCOA) and electronic patient reported outcomes (ePRO) in clinical trials brings measurable improvements in data quality, operational efficiency, and patient engagement. Whether used independently or together through a unified platform like TrialKit, these digital tools streamline study workflows and support more reliable, patient-centric research.
Improved Data Accuracy and Compliance
- Eliminates errors associated with paper-based data collection and transcription
- Enables real-time data validation and automated edit checks
- Ensures regulatory compliance with FDA, EMA, and 21 CFR Part 11 standards
- Reduces discrepancies by capturing clinical outcome data directly from patients, clinicians, and caregivers
Enhanced Patient Engagement and Retention
- Offers a mobile-friendly interface that empowers patients to report outcomes on their own schedule and device
- Sends automated alerts and reminders to boost compliance and reduce missed entries
- Supports remote data collection, reducing site burden and making participation more convenient, especially in decentralized clinical trials
Streamlined Clinical Trial Workflows
- Fully integrates with TrialKit’s mobile and web-based platform for seamless data capture and monitoring
- Enables real-time access to outcome data for sites, sponsors, and CROs
- Minimizes manual data entry and administrative burden by automating routine processes such as reminders, data syncing, and audit trails



