Challenge Series – Driving Customer Profitability with Enterprise Model eClinical Platform and Pricing
Developing new drugs is extremely expensive and costs are not going down. In 2022, the consulting firm Deloitte found the average cost to develop a single new drug among leading pharmaceutical companies increased by nearly $300 million over the previous year to a total of approximately $2.3 billion.1 So, it is no mystery why clinical research sponsors invest so much money in the development of potential blockbusters. Without these super-profitable medicines, research into lesser-known, but critically important therapies would not be possible.
The challenge is finding ways to allow sponsors to run more trials – ranging from smaller rare disease therapies all the way up to blockbusters – more cheaply and efficiently. Doing so would allow pharmaceutical, biotech, and device companies to bring more products to market faster while helping many more patients. Crucial Data Solutions (CDS) believes it offers a solution – one that provides the user flexibility, product depth, and advanced technology at a value point that can help life science companies improve the business results of research and facilitate an increase in the number of studies. In this paper, we will explore how the solutions and approaches offered by CDS can help different research stakeholders conduct more cost-efficient clinical trials.
Step One – Adopting the Right Research Technology Platform
Clinical research professionals typically rely upon the mixing and matching of a variety of software applications and platforms to gain the optimal functionality and price points necessary to manage study data. Each facet of research – recruitment, study design, collection, and management of patient data, etc. – often comes with its own application. This adds unnecessary complexity and labor burden across all research stakeholders. However, having one platform that allows teams to do more with existing resources can unlock efficiencies and reduce costs. For example, the ability for study teams to access and analyze all study data from a single database helps save time and money while enabling patient-facing study team members to focus on engagement activities and other critical tasks.
The variety across eClinical platforms is best demonstrated by a GAP analysis of platform applications that can be deployed in either the web or mobile environments, but not both. TriaKit is the only unified SaaS platform that supports both environments with the additional benefit of an intuitive, user-friendly approach that empowers research teams to be self-sufficient. Study teams can reduce time delays with TrialKit’s drag-and-drop design tool for building, managing, and modifying study-related forms and study databases that TrialKit was designed to manage. Any TrialKit user (study team, sponsor, site personnel and study participants) can use the web and/or the mobile version of TrialKit available in iOS or Android to manage most, if not all, aspects of their role throughout the clinical trial process.
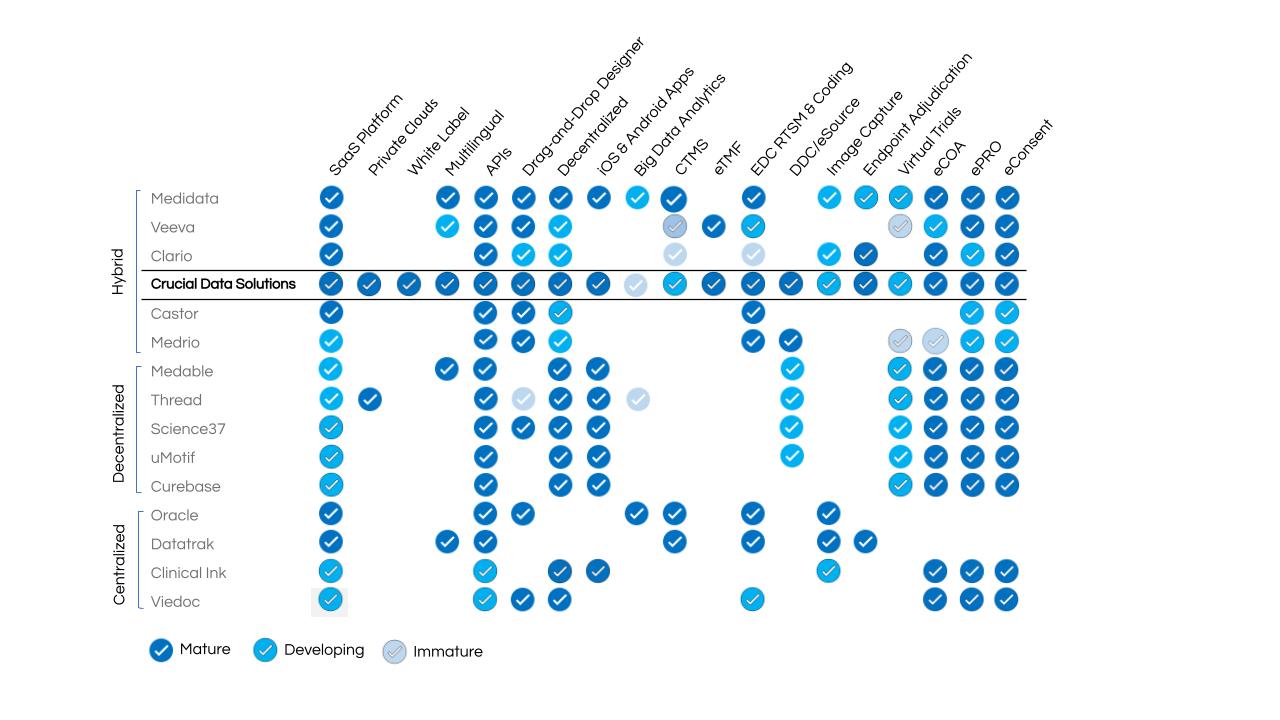
Stakeholder Benefits of One, Flexible Study Platform
Sponsors
A platform such as TrialKit helps sponsors more effectively distill multiple study activities into one centralized access point. This helps to eliminate the cost of selecting, integrating, and supporting multiple platforms that are often required to meet the unique requirements of each individual protocol. TrialKit is also easy to scale across a sponsor’s entire portfolio of studies, and doing so can unlock significant volume discounts (in excess of 50 percent per study).
CROs
Contract research organizations (CROs) also benefit from TrialKit’s flexibility and scalability. The ability to do more with the resources they have helps to maximize the value they can offer sponsors while maintaining higher margins, particularly for professional services.
eClinical Vendors
For companies providing eClinical technology and/or services (e.g., eCOA, eConsent, etc.,) TrialKit can help them to differentiate themselves and compete more effectively for business from sponsors and/or CROs. By seamlessly integrating TrialKit’s web and mobile functionality into their existing offering, with white-labeling if desired, eClinical vendors are able to present a more comprehensive and wide-reaching suite of technology and services.
Pricing Matters
Using a platform like TrialKit can enable any sponsor to increase the size of their study portfolios; however, per study spending for your eClinical platform can make scaling up a budget a bit of a challenge. Shifting to an enterprise pricing model, such as the one CDS offers, can help protect margins. Subscription pricing allows researchers to scale operations while removing the risk of unplanned expenses that come with inevitable mid-study changes.
Stakeholder Benefits of Enterprise Pricing
Sponsors
For sponsors with large and growing portfolios of studies, enterprise pricing allows for more predictable budgeting and fewer expense surprises. It also reduces the costs of selecting, integrating, and supporting the multiple disparate software platforms that are commonplace when supporting modern clinical trials. This is especially true for decentralized clinical trials (DCTs) that require numerous, non-integrated tools for collecting secure and accurate data remotely.
CROs
CROs win business by largely providing services that offer value and peace of mind to their sponsor clients. One sure way to diminish a customer’s peace of mind is through frequent change orders and increased costs over what was originally quoted. With TrialKit, CROs can issue accurate cost proposals and earn long-term loyalty through a combination of fair pricing and expert professional services.
eClinical Vendors
Similar to CROs, enterprise pricing with TrialKit allows providers of eClinical technologies to create highly accurate cost proposals. When eClinical vendors augment their existing capabilities with TrialKit’s unique web and mobile platform, they can fill technology gaps while minimizing the cost to their customers. Choosing an enterprise subscription strategy also allows eClinical vendors to eliminate the need for new scopes of work (SOWs) and/or software license agreements for different technology requirements for each new study, or fees related to a protocol amendment or mid-study change. Furthermore, eClinical vendors can optimize their team’s productivity by enabling them to provision and configure studies on their own, eliminating the need for expensive third-party assistance in many cases.
The Flexibility to Grow
Growth, when referring to clinical trials, is not limited to the volume of clinical trials. It can also refer to expanding access to clinical research for all patients. Everyone working in the research space has been called to action in order to increase patient diversity and enroll more representative populations of patients. In the United States, the Food and Drug Omnibus Reform Act (FDORA) made it a requirement that trial sponsors develop diversity action plans for each study2 as a way to help ensure widespread safety and efficacy of new therapies.
TrialKit helps facilitate efforts to build more diverse populations of research participants in a number of ways. First, its ability to seamlessly integrate decentralized clinical trial (DCT) technologies allows study teams to deploy remote data collection techniques to even the most remote parts of the world. Further, the TrialKit platform addresses the diverse language requirements of global studies, with its translatable web and mobile interface, enabling the support of any foreign language requirements. This makes localizing study materials simple, allowing researchers to engage with patients regardless of where they live.
All of TrialKit’s Enterprise capabilities are powered by dedicated, CDS-managed private servers hosted in Amazon Web Services facilities. This ensures optimal speed and performance at all times, dedicated processing power, unlimited data storage, and the ability to scale in support of any study or portfolio of studies.
Stakeholder Benefits of a Platform Built for Growth
Sponsors
In addition to facilitating the efforts dictated in their diversity action plans, adopting a unified (web and mobile) platform allows sponsors to conduct both traditional trials and DCTs faster, and at lower cost. TrialKit’s configurable, proprietary templates and easy-to-use workflows allow sponsor teams to leverage in-house resources to manage TrialKit components that include EDC, eSource, eCOA, ePRO, RTSM, eTMF, and eConsent, allowing their partners to focus on higher value services.
With the growth of DCT applications in clinical trials, TrialKit gives sponsor teams the power to develop and validate new digital endpoints in DCTs without third-party assistance.
CROs
In addition to enjoying many of the same growth-related benefits as sponsors, CROs can also use the TrialKit platform to maximize the value of what they do best: provide expert professional services. Their adoption of the unified web and mobile platform can simplify many tasks and allow CRO leaders to focus on consulting and optimizing study operations for their clients. CROs can also build new lines of revenue by using TrialKit to configure and integrate eTMF, EDC, eSource, wearable device data, eCOA, ePRO, RTSM and eConsent functionality with standardized, proprietary templates and workflows.
eClinical Vendors
TrialKit provides eClinical vendors with advanced platform capabilities, including features that allow for easy integration of DCT approaches like remote data collection via ePRO, eConsent, virtual visits, and more. eClinical companies are able to better position themselves as difference-makers when it comes to helping their customers meet the requirements for designing and conducting more diverse clinical trials. These same features of TrialKit mean that eClinical vendors can grow their capabilities to develop and validate DCT templates, forms, and digital endpoints. Like CROs, eClinical vendors can also take advantage of TrialKit to configure and integrate other products and platforms, potentially creating new sources of revenue.
Conclusion
The key to accelerating the development of safe, effective therapies, all while improving clinical trial diversity, is utilizing technologies that allow researchers to do more with less. TrialKit offers the flexibility researchers need, regardless of role (sponsor, CRO, eClinical vendor) to design and conduct any type of study – ranging from small, regional, centralized studies to large, global DCTs. The enterprise approach to pricing allows customers to better control costs and protect margins, eliminating the need for frequent change orders and the accompanying unplanned expenses. The platform’s integration features allow users to get the most out of existing resources, further reducing costs. The combination of platform flexibility and cost allows customers the ability to grow and scale their capabilities as needed.
For more information, visit crucialdatasolutions.com
1 Philippidis, A. (2023, June 9). The unbearable cost of drug development: Deloitte Report shows 15% jump in R& D to $2.3 billion. GEN. https://www.genengnews.com/gen-edge/the-unbearable-cost-of-drug-development-deloitte-report-shows-15-jump-in-rd-to-2-3-billion/#:~:text=Deloitte%20found%20that%20the%20average,clinical%20trials%20to%20the%20market.
2 Baumann, J. (2023, January 19). Diversity in clinical trials at FDA gets a boost from new law. Bloomberg Law. https://news.bloomberglaw.com/pharma-and-life-sciences/diversity-in-clinical-trials-at-fda-gets-a-boost-from-new-law
