-
Has COVID-19 Become the Catalyst for Virtual Clinical Trials?
A new wave of interest in virtual trials has been generated by the coronavirus pandemic. But will the enthusiasm remain after the conclusion of the pandemic? In this post, we provide our perspective on why the virtual trial may be here to stay.
-
4 Best Practices in TrialKit for Greater Patient Engagement
Making patient engagement more achievable will lead to more successful clinical trials. In this post, we detail four best practices in the TrialKit platform to help research teams reach optimal patient engagement.
-
How TrialKit Can Aid in Virtual Clinical Trials
With growing interest in virtual trials comes the need for technology to support it. Keep reading to learn how TrialKit’s inherent design is ideal for data collection and study management in the virtual trial space.
-
What are the Benefits of Private Cloud Storage in Clinical Research?
Private clouds are quickly becoming an alternative to typical cloud storage for clinical trial data. If you’re wondering what sets the private cloud apart, this blog post breaks down its various benefits.
-
Misconceptions about Bring Your Own Device (BYOD) Technology in Clinical Trials
A newer technique to the industry, bring your own device is approached with apprehension. In this post, we’ve identified four misconceptions about BYOD and provided explanations on each.
-
8 Elements Your RTSM System Should Have
How do you optimize and unify your randomization and supply management workflow? Dive into this post to learn which functions your RTSM system should have.
-
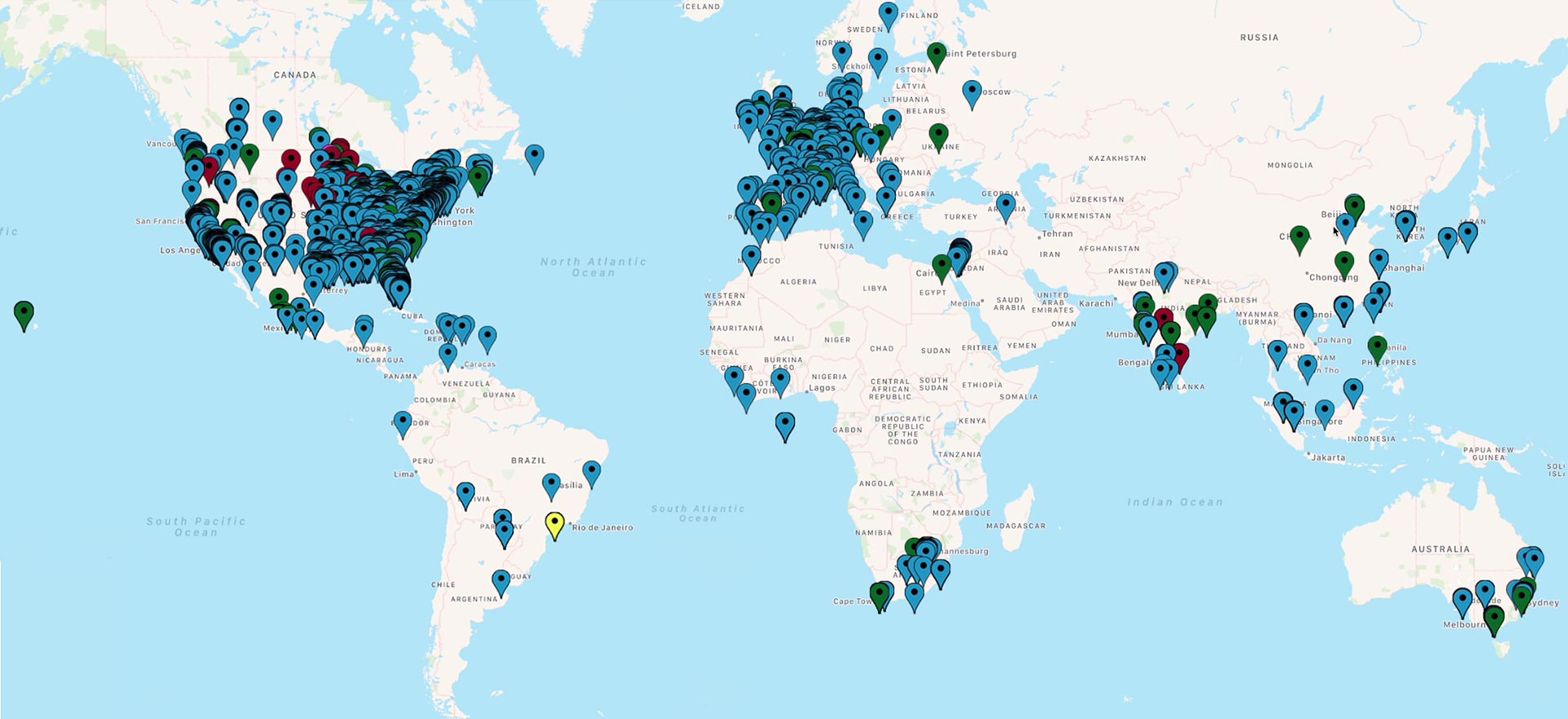
Crucial Data Solutions’ eClinical Platform Reaches 10,000 Users
The first quarter of 2019 has brought about a meaningful milestone for Crucial Data Solutions. Users of TrialKit’s web-based eClinical platform and native mobile app have surpassed the 10,000 mark.
-
Improve Data Collection with These Tips for Electronic Case Report Form (eCRF) Design
In this post, we reveal some tips and techniques for designing powerful and purposeful CRFs that fuel accurate data collection.
-
Recapping 2018 at Crucial Data Solutions
As we bid farewell to 2018 and begin rounding the corner to 2019, we find ourselves reflecting on all we’ve achieved this year. Our team enjoyed supporting research professionals worldwide as they built studies, deployed to sites around the globe, and continued to efficiently collect data using the platform’s latest technologies.